Hereditary Angioedema Mechanism of Disease

Most cases of hereditary angioedema (HAE) are caused by a deficiency or dysfunction of C1 esterase inhibitor (C1-INH), resulting in the inability to regulate the contact system. C1-INH inhibits plasma kallikrein and coagulation factor XIIa. In the absence of functional C1-INH, activated factor XII (factor XIIa) activates prekallikrein to form kallikrein, which can then rapidly activate factor XII, resulting in a positive feedback loop.1-3
Increased bradykinin levels trigger an HAE attack2,4
Kallikrein-kinin system
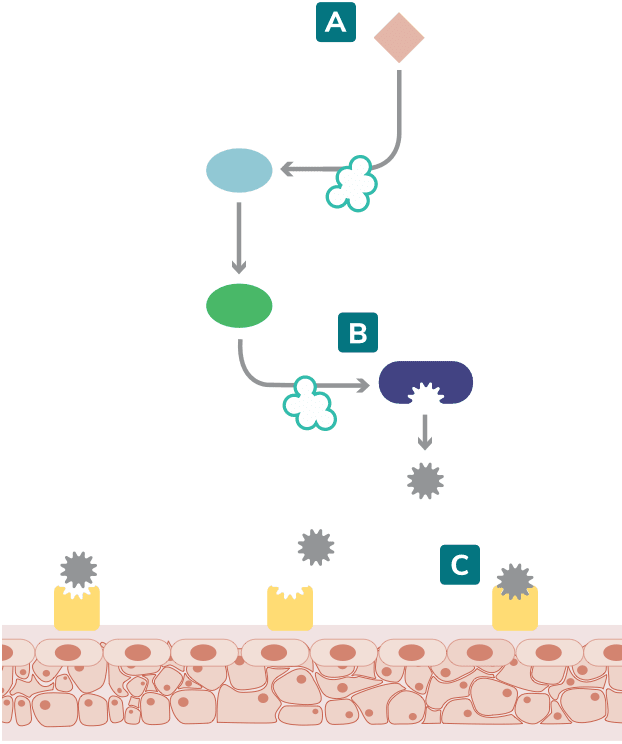
Adapted from Zuraw 2008 and Kaplan 2010.1,3
HAE attacks are initiated when the kallikrein-kinin cascade is activated. 3,5
In response to this activation, plasma kallikrein activity increases the cleavage of high-molecular-weight kininogen (HMWK) to produce bradykinin. 3,4
Excessive bradykinin, after binding to bradykinin B2 receptors, causes an increase in blood vessel permeability. This allows fluid to pass through the blood vessel walls, causing subcutaneous or submucosal swelling.2-4
In the classical complement pathway, insufficient or defective C1-INH also results in diminished levels of proteins such as C4, a useful biomarker in the diagnosis of HAE.1,2
-
Factor XIIa
-
Pre-kallikrein
-
High-Molecular-Weight Kininogen
-
Bradykinin
-
Vascular cells
-
Kallikrein
-
Missing/dysfunctional C1-INH
-
Bradykinin B2 Receptor
Types of HAE
The two most common types of HAE are Type I and Type II. While similar in clinical presentation, they result from different C1-INH mutations.1

~85% of patients have Type I, which is characterized by a deficiency in C1-INH1
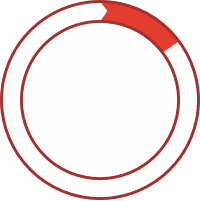
~15% of patients have Type II, which is characterized by dysfunctional C1-INH1
HAE Types I and II are caused by a genetic mutation in the SERPING1 gene, which codes for C1-INH.6
There is another type of HAE—HAE with normal C1-INH (formerly known as Type III)—that has the same presentation as Types I and II, but with normal levels and function of C1-INH.6
Genetic aspect
HAE is an autosomal dominant genetic disorder, so a person with HAE has a 50% chance of passing it down to offspring.7
Most cases of HAE are inherited5:
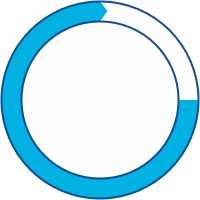
75% of patients inherit HAE

MUTATION
25% of patients have a spontaneous genetic mutation
HAE can affect both adults and children, with symptoms generally worsening after puberty.4
Regardless of the frequency and severity of their own attacks, many patients worry or experience anxiety about possibly passing HAE down to their children.8,*
Have questions?
To learn more about the causes of HAE, submit a request and a Takeda representative will get in touch with you.
aBased on a 2017 quality-of-life survey of 445 HAE patients.8
